Carboxy-PTIO

NO Detection
-
Product codeC348 Carboxy-PTIO
-
CAS No.148819-93-6
-
Chemical name2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, sodium salt
-
MWC14H16N2NaO4=299.28
| Unit size | Price | Item Code |
|---|---|---|
| 10 mg | C348-10 |
Product Description
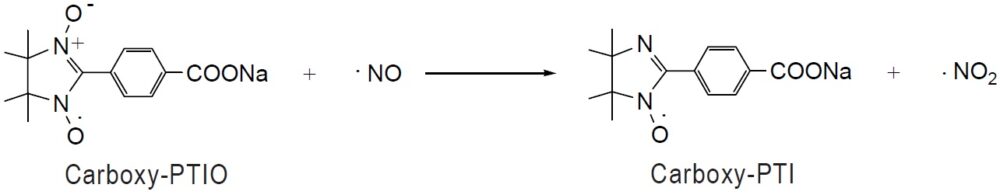
Carboxy-PTIO is a stable, water-soluble organic radical that reacts with NO to form NO2· This reaction can be monitored by electron spin resonance (ESR). NO is an unstable molecule and has a complex reaction cascade for its metabolism in biological systems. Rapidly generated NO-related metabolites carry out various physiological activities. Commonly used NO scavengers such as hemoglobin trap NO; they also trap NOS inhibitors such as arginine derivatives. These NO scavengers also quench all other NO-related metabolites at the same time. In contrast, Carboxy-PTIO does not dramatically affect other NO-related product systems because it transforms NO to NO2, which is a metabolite of NO. Thus, Carboxy-PTIO can be used to investigate the effects of NO separately from its downstream metabolites. Dr. Akaike and others showed that Carboxy-PTIO suppresses relaxation of the rat aorta ring, which is induced by acetylcholine, twice as effectively as NG-nitroarginine. Dr. Yoshida and others reported that downstream metabolites of NO, generated by treatment with Carboxy-PTIO, have an increased antiviral activity compared to NO alone. The NO metabolites play important roles in biological systems; therefore, they should be investigated separately from NO.
Reaction of NO quenching
Technical info
0.4 mg/mL(20 mmol/L; HEPES buffer, pH 7.0), 10 mg/mL(33 mmol/L; Phosphate Buffer, pH7.4)
References
1. E. F. Ullman, et al., Studies of Stable Free Radicals. X. Nitronyl Nitroxide Monoradicals and Biradicals as Possible Small Molecule Spin Labels. J Am Chem Soc. 1972;94:7049-7059.
2. Y. Miura, et al., Polymers Containing Stable Free Raficals, 5. Preparation of a Polymer Containing Imidazoline 3-Oxide 1-Oxyl Groups. Macromol Chem Phys. 1973;172:233-236.
3. K. Inoue, et al., Magnetic Properties of the Crystals of p-(1-Oxyl-3-Oxide-4, 4, 5, 5-Tetramethyl-2-Imidazolin-2-Yl)Benzoic acid and Its Alkali Metal Salts. Chem Phys Lett. 1993;207:551-555.
4. T. Akaike, et al., Antagonistic Action of Imidazolineoxyl N-Oxides Against Endothelium-Derived Relaxing Factor/NO Through a Radical Reaction. Biochemistry. 1993;32:827-832.
5. J. Joseph, et al., Trapping of Nitric Oxide by Nitronyl Nitroxides: an Electron Spin Resonance Investigation. Biochem Biophys Res Commun. 1993;192:926-934.
6. M. Yoshida, et al., Therapeutic Effects of Imidazolineoxyl N-Oxide Against Endotoxin Shock Through Its Direct Nitric Oxide-scavenging Activity. Biochem Biophys Res Commun. 1994;202:923-930.
7. T. Az-Ma, et al., Reaction Between Imidazolineoxil N-Oxide(Carboxy-PTIO) and Nitric Oxide Released from Cultured Endothelial Cells:Quantitative Measurement of Nitric Oxide by ESR Spectrometry. Life Sci. 1994;54:PL185-PL190.
8. H. Maeda, et al., Multiple Functions of Nitric Oxide in Pathophysiology and Microbiology: Analysis by a New Nitric Oxide Scavenger. J Leukoc Biol. 1994;56:588-592.
9. T. Akaike, et al., Quantitation of Nitric Oxide Using 2-Phenyl-4, 4, 5, 5-Tetramethylimidazoline-1-Oxyl 3-Oxide(PTIO). Methods Enzymol. 1996;268:211-221.
10. S. Satoh, et al., NO Donors Stimulate Noradrenaline Release from Rat Hippocampus in a Calmodulin-dependent Manner in the Presence of LCysteine. J Cell Physiol. 1996;169:87-96.
11. D. C. Hooper, et al., Prevention of Experimental Allergic Encephalomyelitis by Targeting Nitric Oxide and Peroxynitrite: Implications for the Treatment of Multiple Sclerosis. PNAS. 1997;94:2528-2533.
12. S. Pfeiffer, et al., Interference of carboxy-PTIO with Nitric-Oxide and Peroxynitrite-Mediated Reactions. Free Radic Biol Med. 1997;22:787-794.
Handling and storage condition
| Appearance: | Dark blue to black powder |
|---|---|
| Purity (TLC): | ≧ 97.0 % |
| Solubility in HEPES buffer: | To pass test (clear, purple) |
| Carboxy-PTI Dye content (ESR): | To pass test (Carboxy-PTI ≦5%) |
| -20°C, Protect from light |












