GEDTA(EGTA)

Metal Chelate
-
Product codeG002 GEDTA(EGTA)
-
CAS No.67-42-5
-
Chemical nameO,O'-Bis(2-aminoethyl)ethyleneglycol-N,N,N',N'-tetraacetic acid
-
MWC14H24N2O10=380.35
| Unit size | Price | Item Code |
|---|---|---|
| 100 g | G002-12 |
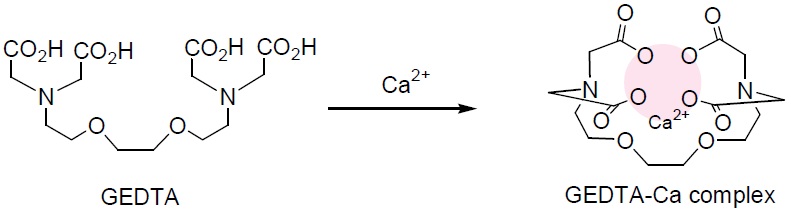
Calcium Chelation
Product Description
EGTA is the most widely used calcium- selective chelator. The calcium complex of EGTA is 100,000 times more stable than its Mg complex. It is utilized to prepare calcium buffers and control the calcium ion concentration.
References
1. C. L. Luke, et al., Photometric Determination of Magnesium in Electronic Nickel. Anal Chem. 1954;26:1778-1780.
2. F. S. Sadek, et al., Visual EGTA Titration of Calcium in Thepresence of Magnesium. Talanta. 1959;2:38-51.
3. R. Pribil, et al., Contributions to the Basic Problems of Complexometry-XX Determination of Calcium and Magnesium. Talanta. 1966;13:233-236.
4. T. Tatsumi, et al., Hypochlorous Acid Mobilizes Intracellular Zinc in Isolated Rat Heart Myocytes. J Mol Cell Cardiol. 1994;26:471-479.
5. H. Ohata, et al., Confocal Imaging Analysis of ATP-Induced Ca2+ Response in Individual Endothelial Cells of the Artery in Situ. Am J Physiol. 1997;272:C1980-C1987.
6. I. Sakabe, et al., Induction of Apoptosis in Neuro-2A Cells by Zn2+ Chelating. Cell Struct Funct. 1998;23:95-99.
Handling and storage condition
| Appearance: | White crystalline powder |
|---|---|
| Purity (Titration): | ≧ 97.0 % |
| Solubility in NaOH solution: | To pass test (clear, colorless) |
| Sulfated ash: | ≦ 0.10 % |
| Heavy metals (as Pb): | ≦ 0.001 % |
| Iron (Fe): | ≦ 0.001 % |
| Ambient temperature |









