-SulfoBiotics- Protein Redox State Monitoring Kit Plus

Biosulfur Analysis
-
Product codeSB12 -SulfoBiotics- Protein Redox State Monitoring Kit Plus
| Unit size | Price | Item Code |
|---|---|---|
| 20 samples | SB12-12 |
| 20 samples | ・Protein-SHifter Plus ・Reaction Buffer ・Reaction Buffer ・Lysis Buffer |
×20 ×4 ×4 ×4 |
|---|
Product Description
Protein thiol is a major redox signaling in the cellular processes. Modification of protein thiol by oxidative stress associate with the redox state in the cells. The approach of this kit is detecting the number of thiol residues accurately in order to study the redox state in oxidative stress environment.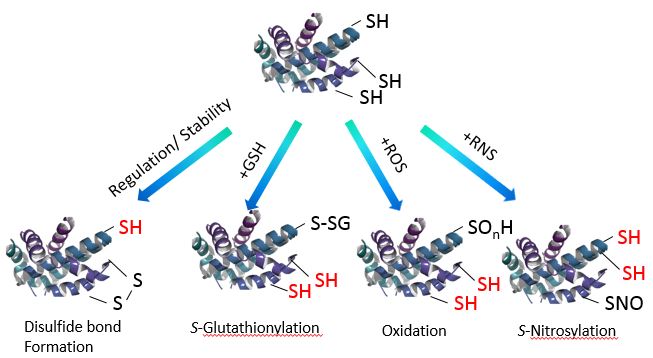
Fig. 1 Redox States of Thiol group(s) in Protein
Mechanism of Thiol Modification with Protein-SHifter
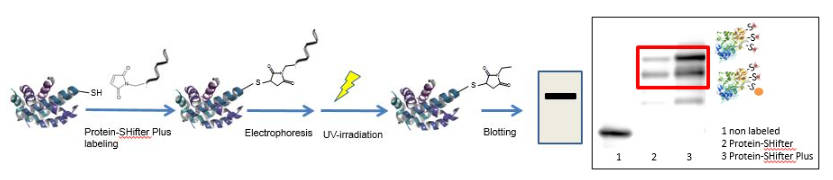
Fig. 2 Maleimido group of the Protein-SHifter binds to a free thiol group of a protein. The conjugate consists of a unique molecular weight that shifts properly in electrophoresis. Photocleavage method enhances membrane transcription efficiency in western blotting.
Visualization of Redox State of Thiol Residues in a Protein
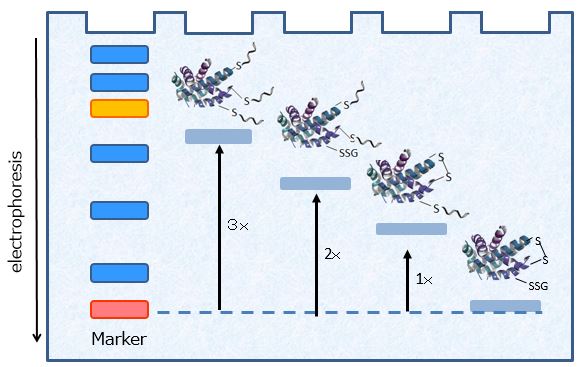
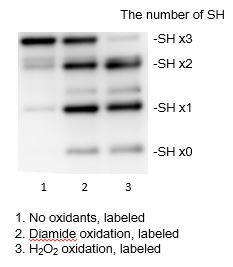
Fig. 3 One molecule of Protein-SHifter binds to one molecule of free-thiol in protein. The number of the conjugation is directly proportional to the number of free thiol as clear band-shift.
| Developer | Dojindo Molecular Technologies, Inc. |
|---|
Manual
Technical info
References
1) S. Hara, T. Nojima, K. Seio, M. Yoshida and T. Hisabori, "DNA-maleimide: An improved maleimide compound for electrophoresis-based titration of reactive thiols in a specific protein" Biochim. Biophys. Acta, 2013, 1830(4), 3077.
2) S. Hara, Y. Tatenaka, Y. Ohuchi and T. Hisabori, "Direct determination of the redox status of cysteine residues in proteins in vivo", Biochem. Biophys. Res. Commun., 2015, 456, (1), 339. ![]()
3) Y. J. Suzuki, L. Marcocci, T. Shimomura, Y. Tatenaka, Y. Ohuchi and T. I. Brelidze, 'Protein Redox State Monitoring Studies of Thiol Reactivity', antioxidant., 2019, 8, (5), 143.
Q & A
-
Q
What is the adequate concentration of proteins and thiols in my sample?
-
A
Adjust the protein concentration about 0.1 to 1 mg / mL and the thiol concentration to 100 μmol / mL or less.
Please refer to the following protocol for a quantification example of thiol concentration (for 96 well plate) using DTNB (product code: D029).
1. Reagent and solution preparation
Required material: Reaction buffer (100 mmol / L sodium phosphate, pH 8.0, 1 mmol / L EDTA)
A) 400 μmol / L DTNB solution
(1) Weigh 4 mg DTNB [5,5′-Dithiobis (2-nitrobenzoic acid), Code: D029] and dissolve in 1 mL of reaction buffer. (10 mmol / L) solution (a)
(2) Dilute the solution (a) 25 times with reaction buffer. Final concentration: 400 μmol / L
* Prepare 0.1 mL of solution for each well. (ex. if you use 9 wells, please prepare about 1mL.)
B) L-Cysteine standard solution
(1) Dissolve 2.42 mg of L-Cysteine in 5 mL of reaction buffer to make 4 mmol / L of L-Cysteine solution. Solution (b)
(2) Dilute 100 μL of Solution (b) with 900 μL of reaction buffer to make a 400 μmol / L of L-Cysteine solution. Dilute this solution twice in order to make L-Cysteine standard solution (200, 100, 50, 25, 12.5, 0 μmol / L).
C) Measurement sample
Prepare several samples (cell lysate, protein solution, etc.) diluted with reaction buffer. (To measure at N = 3, prepare 350 μL or more.)
2. Procedure
(1) Add 100 μL of the measurement sample and L-Cysteine standard solution to each well of a 96-well microplate (Figure 1).
(2) Add 100 μL of 400 μmol / L DTNB solution and mix by pipetting.
(3) After incubating at room temperature for 15 minutes, measure the absorbance at 412 nm with a microplate reader.
(4) Calculate the thiol concentration in the measurement sample from the L-Cysteine calibration curve (Figure 2).
*Calculate the thiol concentration in the sample from the sample dilution ratio.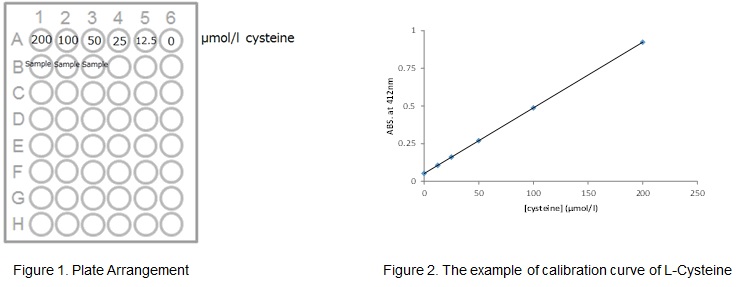
Handling and storage condition
| 0-5°C, Protect from moisture | |
|
Danger / harmful symbol mark |
  
|
|---|---|











