Cystine Uptake Assay Kit

Cystine Uptake Assay Kit
- Easier way to cystine uptake assay
- Applied for plate assay
-
Product codeUP05 Cystine Uptake Assay Kit
| Unit size | Price | Item Code |
|---|---|---|
| 20 tests | UP05-10 | |
| 100 tests | UP05-12 |
<Approximate usage> One plate of 96-well plate.
| 20 tests | Cystine Analog Solution Fluorescent Probe Reducing Agent Assay Buffer |
45 μl×1 ×1 ×1 5 ml×1 |
|---|---|---|
| 100 tests | Cystine Analog Solution Fluorescent Probe Reducing Agent Assay Buffer |
225 μl×1 ×1 ×2 25 ml×1 |
Description
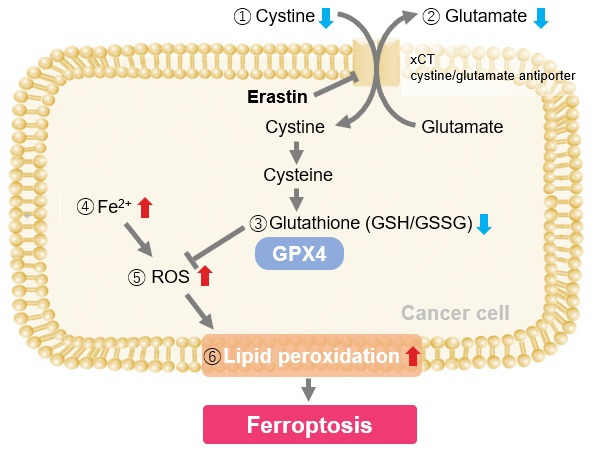
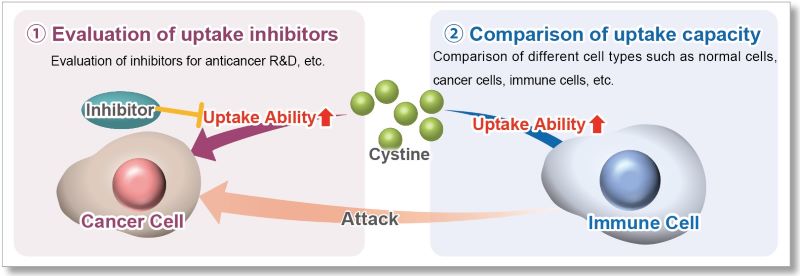
The cystine transporter xCT is a crucial transporter that maintains cellular redox balance by regulating glutathione synthesis via cystine uptake. xCT is highly expressed in several types of cancer cells and is expected to be a therapeutic target for cancer.
Therefore, xCT has recently attracted researchers' attention as one of the targets for cancer treatment. The xCT inhibitors sulfasalazine and erastin are known to reduce intracellular glutathione levels by inhibiting cystine uptake, thereby inducing ferroptosis, a form of cell death. It is also known that immune cells highly express xCT upon activation, suggesting that intracellular redox regulation via cystine uptake is also important for immune responses.

Manual
Technical info
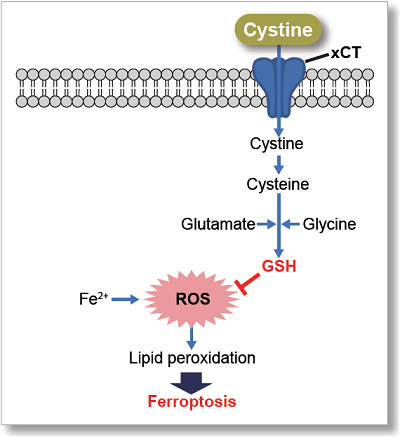
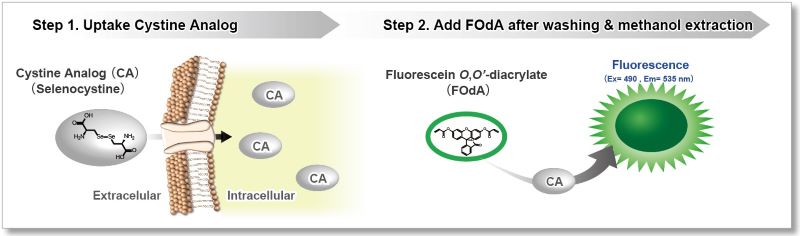
This kit uses Selenocystine as a Cystine Analog (CA) and can measure the cystine uptake ability of cells by a plate reader in a short time.
Principle
The Cystine Analog (CA) in this kit can be taken up into cells via xCT, and the incorporated CA can be specifically detected using the Fluorescent Probe and Reducing Agent. Thus, the xCT activity can be measured easily.[Patent applied]
The relevant technicals are published in the following journal:
Shimomura T, Hirakawa N, Ohuchi Y, Ishiyama M, Shiga M, Ueno Y, Simple Fluorescence Assay for Cystine Uptake via the xCT in Cells Using Selenocystine and a Fluorescent Probe. ACS Sensors, 2021, 6(6), 2125-2128
Application Data 1: Evaluation of xCT inhibitor Sulfasalazine or Erastin)
Using this kit, we measured the inhibitory effect of sulfasalazine and erastin on cystine uptake by HeLa cells.
The fluorescence intensity of the sulfasalazine and elastin groups decreased significantly, indicating that both reagents inhibit cystine uptake.

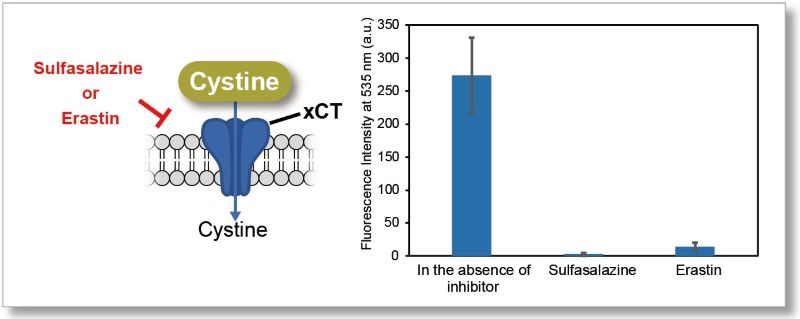
Experiment Condiitons
Cell Line: HeLa cells
Pretreatment: DMEM (cystine-free, serum-free), 37℃, 5 min
Uptake conditions:0.5 mmol/l sulfasalazine or 2 μmol/l erastin / Cystine Analog / DMEM (cystine-free, serum-free), 37℃, 30 min
Instrument: Fluorescent Plate Reader
Filter: Ex=485 nm, Em=535 nm
Application Data 2: Comparison with Radio Isotope Method
Cystine uptake assay, which used to require RI measurement, can now be easily measured with a plate reader. We compared the uptake in Wild-type (WT), xCT-knockout (KO), and xCT-overexpressed (OE) cells, using Cystine Uptake Assay Kit and Radio Isotope method using [14C]-labeled cystine. As a result, in both cases, the uptake was decreased in KO and increased in OE compared to WT. Therefore, it was confirmed that this product correlated well with the Radio Isotope method.
*This data was kindly provided by Professor Hideyo Sato and Assistant Professor Mami Sato, Laboratory of Biochemistry and Molecular Biology, Department of Medical Technology, Faculty of Medicine, Niigata University.
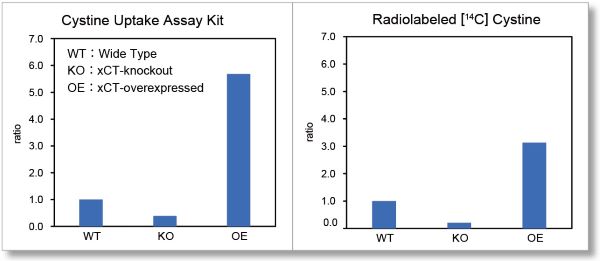
(Using WT's result as a benchmark value)
Experimental Conditions
Cell Line: HT1080 cells (wild-type or xCT-knockout or xCT- overexpressed)
○Cystine Uptake Assay Kit
Pretreatment: PBS (+) (cystine-free, 0.1% glucose, 0.01% Mg2+, 0.01% Ca2+), 37 ℃, 5 min
Uptake Conditions: Cystine Analog / PBS(+) (cystine-free, 0.1% glucose, 0.01% Mg2+, 0.01% Ca2+), 37℃, 30 min
○Radio Isotope Method
Pretreatment: None
Uptake Conditions: 0.05 mmol/l cystine + [14C]cystine (7.4 kBq/ml) /PBS(+) (0.1% glucose, 0.01% Mg2+, 0.01% Ca2+), 37℃, 2 min
Application Data 3: Evaluation of intracellular uptake and redox level after treating with Erastin
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.

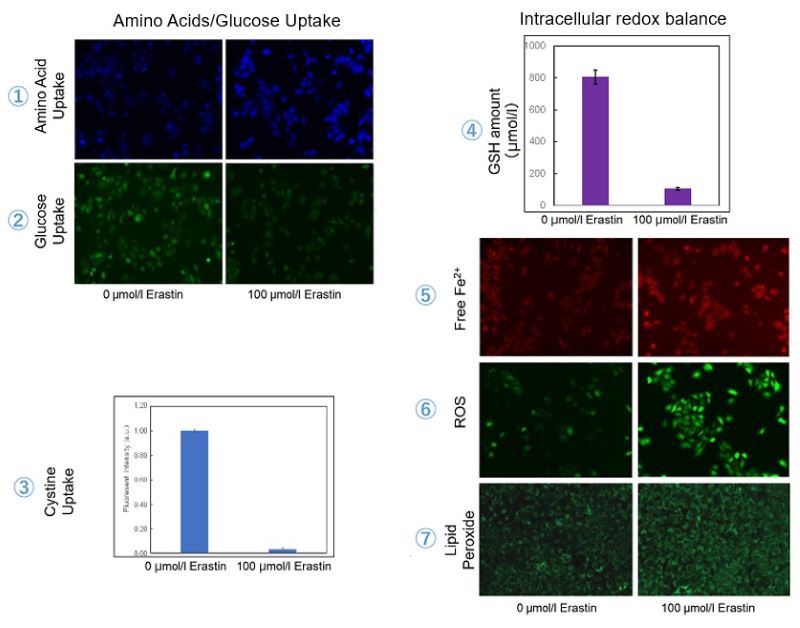
| 1 Amino Acid Uptake | : Amino Acid Uptake Assay Kit (Code: UP04) |
| 2 Glucose Uptake | : Glucose Uptake Assay Kit-Green (Code: UP02) |
| 3 Cystine Uptake | : Cystine Uptake Assay Kit (Code: UP05) |
| 4 Intracellular glutathione | : GSSG/GSH Quantification Kit (Code: G257) |
| 5 Intracellular labile Fe | : FerroOrange (Code: F374) |
| 6 Intracellular total ROS | : ROS Assay Kit -Highly Sensitive DCFH-DA- (Code: R252) |
| 7 Lipid Peroxides | : Liperfluo (Code: L248) |
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
References
| No. | Sample Type | Instrument | Reference (Link) |
|---|---|---|---|
| 1) | Cell (A172; LN229) |
Plate Reader | K. Hayashima, H. Katoh, "Expression of gamma-glutamyltransferase 1 in glioblastoma cells confers resistance to cystine deprivation-induced ferroptosis", J. Biol. Chem., 2022, doi:10.1016/j.jbc.2022.101703. |
| 2) | Cell (HK2; 293T) |
Plate Reader | H. Chen, L. Cao, K. Han, H. Zhang, J. Cui, X. Ma, S. Zhao, C. Zhao, S. Yin, L. Fan, H. Hu, "Patulin disrupts SLC7A11-cystine-cysteine-GSH antioxidant system and promotes renal cell ferroptosis both in vitro and in vivo", 2022, Food Chem. Toxicol., doi: 10.1016/j.fct.2022.113255. |
| 3) | Cell (Hep 3B) |
Plate Reader | X. Hu, Y. He, Z. Han, W. Liu, D. Liu, X. Zhang, L. Chen, L. Qi, L. Chen, Y. Luo, Q. Li, P. Chen, Q. Wu, X. Zhu and H. Guo, "PNO1 inhibits autophagy-mediated ferroptosis by GSH metabolic reprogramming in hepatocellular carcinoma ", 2022, Cell Death Dis., doi:10.1038/s41419-022-05448-7. |
Q & A
-
Q
What kind of microplate can be used for this kit?
-
A
We have tested the following microplate:
Company Product Name Cat No. ibidi μPlate 96 well ibiTreat black S 15 ib89626 AGC techno glass EZVIEW Glass Bottom Culture Plate LB 96well 5866-096 Thermo Fisher 96 Well Black/Clear Bottom Plate, TC Surface, Pack of 10 165305
-
Q
Which transporter uptakes Cystine Analog into the cells?
-
A
Cystine Analog is taken uup via cystine/glutamate transporter (xCT).
-
Q
How many cell lines have been tested by Cystine Uptake Assay Kit?
-
A
The following cell lines have been tested by Cystine Uptake Assay Kit:
A172, A549, A375, HCT116, HepG2, HL60, HT1080, MEF, SBC-5, U-251 MG.
-
Q
Is the Cystine Analog degraded or metabolized after being taken up by cells?
-
A
Cystine Analog is very stable and will not be degraded during the experimental process.
-
Q
Can the cystine uptake solution be stored?
-
A
CA uptake solution cannot be stored. Prepare the required amount of CA uptake solution at the time of use.
-
Q
Can I quantify the cellular cystine uptake ability with this kit?
-
A
No, you can not.
-
Q
If there is no difference in fluorescence intensity between samples or with a blank, what should I check?
-
A
Please check the following five points
1. The number of cell are changed.
Please correct the measured value (fluorescence intensity) by the number of cells or protein concentration.
For details, please refer to FAQ [How to correct the measured value (fluorescence intensity) by the number of cells or protein concentration?] for details.2. Cystine Analog is not fully incorporated into the cells.
Please consider the following time for each operation in the manual as a guide.
・Range of incubation time in cystine-free medium (adherent cells: step 3, floating cell: step 4): 5 - 30 minutes.
・Cystine Analog Incorporation Time (adherent cells step 4, suspension cells step 5): 30 - 60 minutes.3. Unincorporated Cystine Analog by the cells remains in the wells, resulting in an elevated background.
Repeat washing with PBS (adherent cells: step 5, floating cells: step 6).4. The working solution degrades, and the background fluorescent intensity becomes high.
If the working solution is left for a long time after preparation, the fluorescent dye contained in the solution may decompose, leading to an increase in fluorescence intensity. Please add the working solution without delay after preparation.5. Uptake capacity of Cell Type.
Different cell types have significantly different cystine uptake capacities, and cell types with low uptake capacity may exhibit a relatively high background. The figure below illustrates the variations in cystine uptake capacity among different cancer cells. If the high background cannot be resolved using the measures described above, we recommend that you first consider the established cell types listed in the FAQ.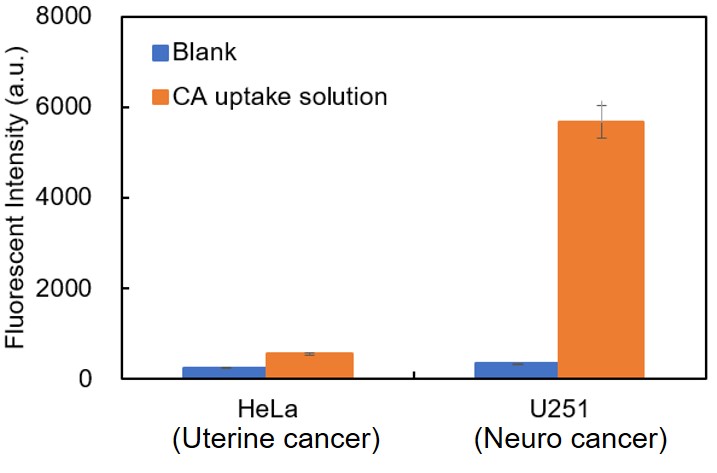
-
Q
Are there any buffers other than cystine-free medium that can be used for the preparation of CA Uptake solution?
-
A
We have used HBSS or PBS with 0.1% Glucose to prepare CA uptake solution when testing HeLa cells.
-
Q
How can I correct the fluorescence intensity by the cell numbers?
-
A
We recommend the following method (see table). The methods of correction, and the appropriate sample solution,
vary depending on whether they are adherent or floating cells. Correct the [measured value by UP05] by referring to the table below.Cells
Adherent cells
Suspension cells
Correction method
Nuclear staining
( Hoechst dye: Fluorometry )Protein Quantification method
(BCA method: colorimetry)Product
Cell Count Normalization Kit [Code:C544]
Sodium bicinchoninate [Code:B037]
Commercial kitsSample preparation
Use sample solutions after performing cystine uptake assay.
Use the remaining portion of the solution from step # 9 in the UP05 instruction manual.
Procedure
Please refer to General Protocol “Culture medium exchange method” in the C544 instruction manual.
Please refer to Experimental example for suspension cells “Inhibition of xCT activity by
erastin (HL60 cells)” in the UP05 instruction manual.Please calculate the fluorescence intensity by correcting the [measured value by UP05] with the [measured value by the correction method].
Corrected fluorescence intensity =[measured value by UP05] /[measured value by the correction method]
※ [measured value by UP05] = (Measured value of sample) - (Measured value of blank)
※ [measured value by the correction method]=(Measured value of sample well) - (Measured value of well without cells)
Handling and storage condition
| 1. Non-medical deleterious substances; 2. Store at -20℃ | |
|
Danger / harmful symbol mark |
   
|
|---|---|