n-Dodecyl-β-D-maltoside

Detergent
-
Product codeD316 n-Dodecyl-β-D-maltoside
-
CAS No.69227-93-6
-
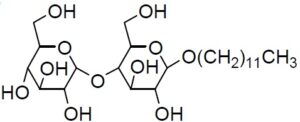
Chemical nameN-Dodecyl-β-D-maltopyranoside
-
MWC24H46O11=510.62
| Unit size | Price | Item Code |
|---|---|---|
| 1 g | D316-10 | |
| 5 g | D316-12 |
Product Description
n-Dodecyl-β-D-maltoside (DDM) is a non-ionic detergent. It has a glyco-chain in its lipophilic site that is similar to the one in n-Octyl-β-D-glucoside. Using this detergent, Dr. Van Aken and others succeeded in solubilizing the active cytochrome oxidase from mitochondria. Its CMC value is 0.17 mM.
Introduction
The phospholipid bilayer is the basic structure of the cell membrane. The most important functions of cells include transportation of substances, energy exchange, and transmission of information. These functions are conducted at the cell membrane by membrane proteins. In membrane biochemistry research, membrane proteins are solubilized and purified to study their structure and function. Proteins bound to cell membranes have hydrophobic sites buried within the phospholipid bilayers and hydrophilic sites facing toward the water layer. Detergents are used to isolate large insoluble molecules such as proteins. Detergents interact with the hydrophobic sites of proteins, which are then solubilized in the water layer, thus separating membrane proteins. It is important to choose a detergent that does not disrupt the bioactivities of target proteins. A detergent requires the following characteristics to be suitable for isolation of
membrane proteins:
1. Sufficient protein solubilization capability
2. No denaturing or inactivation of proteins
3. No interference with protein activities
4. No precipitation at 4ºC
5. Appropriate critical micelle concentrations (CMC) and micelle size
6. No absorption in the UV region
7. No toxicity
8. Availability of detergent detection methods
9. Non-ionic detergent if ion exchange chromatography is used
In the past, polyoxyethylene ether non-ionic detergents were widely used. These detergents, however, had several problems, such as denaturation of proteins and low CMC value, which cannot be separated easily by dialysis. n-Octyl-β-D-glucoside, n-Octyl-β-Dthioglucoside, CHAPS, and CHAPSO eliminate these problems and are widely used today. Most of the current detergents are non-ionic and easily applied to ion exchange chromatography purification. deoxy-BIGCHAP is a non-ionic detergent possessing deoxycholic acid and a gluconamide polar group. It has a high CMC value of 1.4 mM and can be easily separated by dialysis. Because its UV absorbance is low, it can be used for the determination of proteins. deoxy-BIGCHAP has been used for the extraction of opioid receptors from neuroblastoma or hybrid cells of glyoma. It has also been applied to adenylate cyclase or acetyltransferase. These detergents are also widely used to solubilize chromophores or to stabilize enzymes in diagnostic analyses and biochemical assays.
Trials of various kinds of detergents are needed to find the appropriate detergent for each study. Dojindo’s Detergent Screening Sets, which contain assorted packages of detergents, are available for use in these trials.
Chemical Structure
References
1) T. VanAken, S. Foxall-Vanaken, S. Castleman and S. Ferguson-Miller, "Alkyl Glycoside Detergents: Synthesis and Applications to the Study of Membrane Proteins", Methods in Enzymol., 1986, 125, 27.
2)H. Shigematsu, T. Sokabe, R. Danev, M. Tominaga and K. Nagayama, "A 3.5-nm Structure of Rat TRPV4 Cation Channel Revealed by Zernike Phase-contrast Cryoelectron Microscopy", J. Biol. Chem., 2010, 285, 11210.
3)Y. Kofuku, C. Yoshiura, T. Ueda, H. Terasawa, T. Hirai, S. Tominaga, M. Hirose, Y. Maeda, H. Takahashi, Y. Terashima, K. Matsushima and I. Shimada, "Structural Basis of the Interaction between Chemokine Stromal Cell-derived Factor-1/CXCL12 and Its G-protein-coupled Receptor CXCR4", J. Biol. Chem., 2009, 284, 35240.
4)A. J. Kuszak, S. Pitchiaya, J. P. Anand, H. I. Mosberg, N. G. Walter and R. K. Sunahara, "Purification and Functional Reconstitution of Monomeric μ-Opioid Receptors: ALLOSTERIC MODULATION OF AGONIST BINDING BY Gi2", J. Biol. Chem., 2009, 284, 26732.
5)S. Okuda and H. Tokuda, "Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB", PNAS, 2009, 106(14), 5877.
6)B. C. Jennings, M. J. Nadolski, Y. Ling, M. B. Baker, M. L. Harrison, R. J. Deschenes and M. E. Linder, "2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro", J. Lipid Res., 2009, 50, 233.
7)N. Taniguchi and H. Tokuda, "Molecular Events Involved in a Single Cycle of Ligand Transfer from an ATP Binding Cassette Transporter, LolCDE, to a Molecular Chaperone, LolA", J. Biol. Chem., 2008, 283(13), 8538.
Handling and storage condition
| Appearance: | White powder |
|---|---|
| Purity (GC): | ≧ 98.0 % |
| Solubility in water (0.1%): | To pass test (clear, colorless) |
| Solubility in Water (1%): | To pass test (clear, colorless) ≦ 0.100 |
| Specific rotation (20°C): | 46.0 - 50.0°C |
| Water content: | ≦ 1.0 % |
| IR spectrum: | Authentic |
| 0-5°C |











