TES

Good's Buffer
-
Product codeGB18 TES
-
CAS No.7365-44-8
-
Chemical nameN-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid
-
MWC6H15NO6S=229.25
| Unit size | Price | Item Code |
|---|---|---|
| 25 g | GB18-10 | |
| 100 g | GB18-12 |
Introduction
In biological experiments, it is important to maintain the pH of the solutions used. Buffers, mixtures of appropriate weak acids, and their conjugate bases, are usually used. Most biological reactions occur at a neutral pH, from 6 to 8; the buffer needs to be effective in this range. Furthermore, the acids and bases used in the buffer should not produce chelates with metal ions, which are essential in biological systems. For these reasons, Dr. Good developed several aminoethane and aminopropane sulfonic acids that are now widely used for biological research and analysis. Good’s buffers have the following characteristics:
1) High water-solubility
2) Low cell membrane permeability
3) Consistent acid-base dissociation constants
4) Low metal chelating capability
5) High chemical stability
6) Low absorption spectra in UV and visible regions.
Technical info
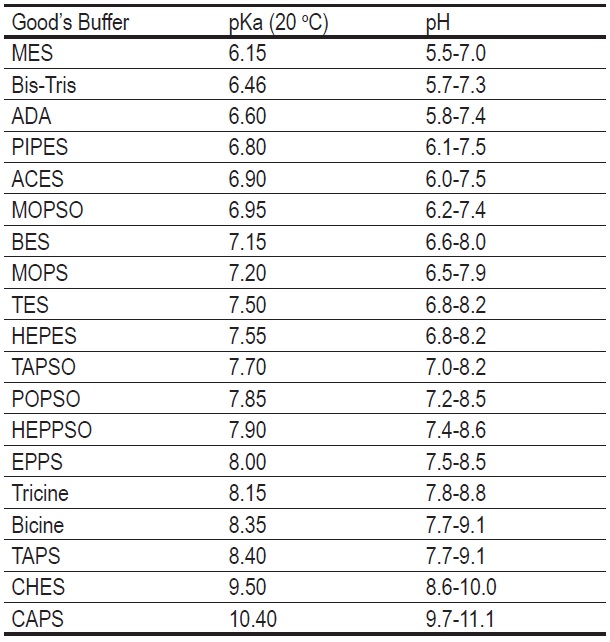
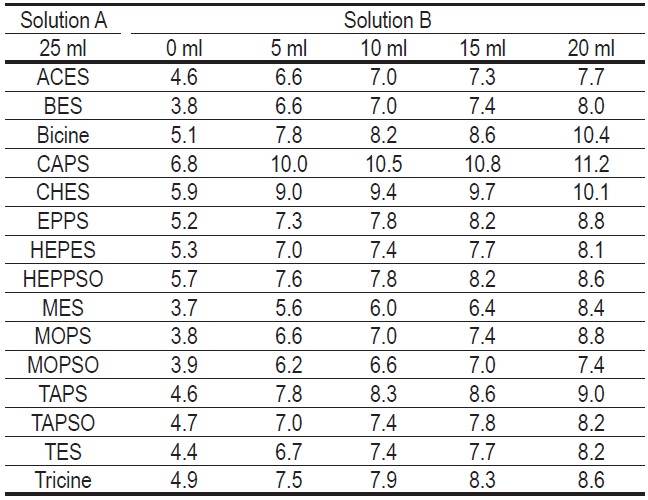
Other Good's Buffer
Solution A: 0.1M Good's Buffer solution
Solution B: 0.1M NaOH solution
References
1. N. E. Good, Uncoupling of the Hill reaction from photophosphorylation by anions. Arch Biochem Biophys. 1962;96:653-661.
2. N. E. Good, et al., Hydrogen ion buffers for biological research. Biochemistry. 1966;5:467-477.
3. C. Ceccarini, et al., Induction and reversal of contact inhibition of growth by pH modification. Nat New Biol. 1971;233:271-273.
4. E. L. Medzon, et al., Substitution of 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES) for bicarbonate in protein-free animal cell culture medium: application to vaccinia virus quantitation and fluorogenic acetylesterase assay in living LM cells. Can J Microbiol. 1971;17:651-653.
5. A. Itagaki, et al., TES and HEPES buffers in mammalian cell cultures and viral studies: problem of carbon dioxide requirement. Exp Cell Res. 1974;83:351-361.
6. W. J. Ferguson, et al., Hydrogen ion buffers for biological research. Anal Biochem. 1980;104:300-310.
7. J. K. Grady, et al., Radicals from “Good’s Ebuffers. Anal Biochem. 1988;173:111-115.
8. J. W. Hanrahan, et al., Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J Membr Biol. 1990;116:65-77.
9. T. Kudo, et al., A simple and improved method to generate human hybridomas. J Immunol Methods. 1991;145:119-125.
Handling and storage condition
| Appearance: | White crystalline powder |
|---|---|
| Purity (Titration): | ≧99.0% |
| Solubility in water: | ≦0.020 (300 nm) |
| Loss on drying (110 °C): | ≦0.40% |
| Sulfated ash: | ≦0.10% |
| Heavy metals (as Pb): | ≦0.0005% |
| Iron (Fe): | ≦0.0005% |
| IR spectrum: | Authentic |
| Ambient temperature |








