DMPO

- Ultra High Purity
- Greater S/N Ratio
- No Pre-purification Required
-
Product codeD048 DMPO
-
CAS No.3317-61-1
-
Chemical name5,5-Dimethyl-1-pyrroline N-oxide
-
MW113.16, C6H11NO
| Unit size | Price | Item Code |
|---|
Description
Product Description
Because of potential cancer risks and their age-promoting effects, free radicals in living bodies have become a frequently studied subject. DMPO is the most frequently used spin-trapping reagent for the study of free radicals. It is suitable for trapping oxygen radicals, especially superoxides, and for producing adducts with characteristic EPR (ESR) patterns. However, most commercially available DMPO contains impurities that cause high backgrounds. Thus, DMPO requires further purification for running experiments on EPR. The quality of Dojindo’s DMPO is well controlled and Dojindo’s DMPO doesn’t require any pre-purification process. There are no impurities to cause a background problem.
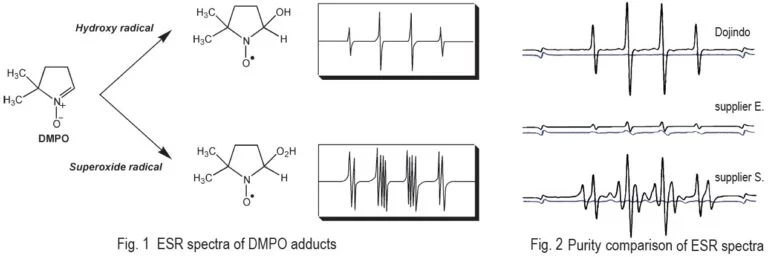
Technical info
General Protocol
Evaluation of superoxide scavenging activities
1. Add 15 μl of DMPO and 50 μl of 5 mM hypoxanthine to 35 μl of 0.1 M Phosphate buffer(pH 7.8).
2. Add 50 μl of SOD standard or samples to be tested and voltex for 1-2 seconds.
3. Add 50 μl of 0.4 U/ml xanthine oxidase and voltex immediately.
4. Transfer the solution to ESR sample tube and measure ESR spectra after certain time of period, e.g. 1minutes.
5. Calculate relative intensity(DMPO-O2-/Mn2+) from the peak height.
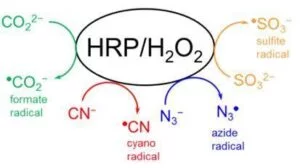
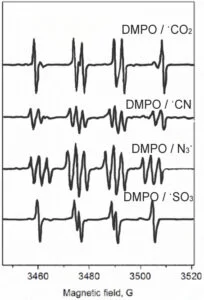
DMPO is capable of trapping not only O-centered radicals but also C-, N-, and S-centered ones. The following experiments are used to demonstrate how different types of radicals can be trapped by DMPO in a simple HRP/H2O2 system by using different protein substrates.
Protocol
1. Prepare a solution of 100 mM phosphate buffer (pH 7.4) containing 25 µM diethylenetriaminepentaacetic acid (DTPA).
2. Make up a solution of the following peroxidase substrates: (A) 100 mM sodium formate (HCOONa); (B) 100 mM potassium cyanide (KCN); (C) 100 mM sodium azide (NaN3); (D) 100 mM sodium sulfite (Na2SO3) in 100 mM phosphate buffer, pH 7.4.
3. Make up a solution of horseradish peroxidase with concentration of 4.0 mg/ml (~ 100 µM) and 1 mM solution of hydrogen peroxide (H2O2).
4. Make up a solution of DMPO with concentration of 1 M.
5. Prepare your reaction mixture to a total reaction volume of 200 µl. Add 130 µl of buffer to an Eppendorf tube.
6. Add 20 µl DMPO of your 1 M DMPO solution, 20 µl of one of the substrates Estock solutions, 10 µl of 1 mM H2O2, and initiate the reaction with 20 µl HRP.
7. Vortex the tube, transfer the solution to a flat cell, and acquire the spectrum.
8. The final concentrations of the components are: 100 mM DMPO, 10 mM substrate (formate, cyanide, azide, sulfite), 50 µM H2O2, and 10 µM HRP.
Data and Protocol were kindly provided by Bruker Corporation.
Superoxide Detection Procedure
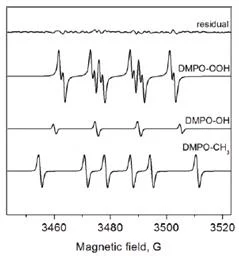
Protocol
1. Prepare a solution of 100 mM phosphate buffer (pH 7.4) containing 25 µM diethylenetriaminepentaacetic acid (DTPA) as transition metal chelator.
2. Make up a solution of 1 mM hypoxanthine in 100 mM phosphate buffer, pH 7.4.
3. Make up a solution of xanthine oxidase with concentration of 1 unit/ml
4. Make up a solution of DMPO with concentration of 1 M.
5. Prepare your reaction mixture to a total reaction volume of 200 µl.
6. Add 70 µl of buffer to an Eppendorf tube. Add 20 µl DMPO of your 1 M DMPO solution and 100 µl hypoxanthine of the stock 1 mM solution.
7. Initiate the reaction with 10 µl xanthine oxidase, vortex the tube and transfer the solution to a flat cell.
8. Insert the flat cell into the cavity, tune the spectrometer, and acquire the spectrum.
The final concentrations of the components are: 100 mM DMPO, 0.5 mM hypoxanthine, and 0.05 units/ml xanthine oxidase.
You should always perform control experiments in which one or more of the reagents are excluded. These experiments will reveal any paramagnetic impurities and will demonstrate that all the components were required to produce the EPR signal.
Data and Protocol were kindly provided by Bruker Corporation.
Purity Data of DMPO by HPLC
No impurities(*) were observed in Dojindo’s DMPO.
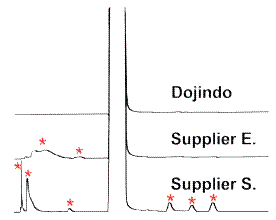
Fig. 2 Comparison of the Purity by HPLC analysis
S/N Ratio in EPR (ESR) Study
Comparison of EPR spectra between Dojindo’s DMPO and other suppliers. Spectra were taken in the presence of hydroxy radicals generated by Fenton reaction(black) and blank(blue). Dojindo’s DMPO gives very clear signal and higher S/N rate than supplier E and S.
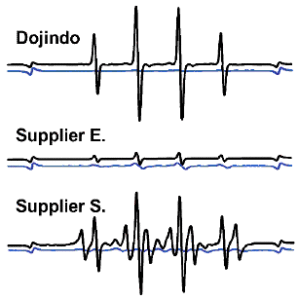
Fig. 3 The signal and background from EPR detection
Immuno-spin Trapping
Description
Immuno-spin Trapping method was developed for detecting DNA and Protein radicals in biological analysis. ROS (Reactive Oxygen Species) produces modification of the structure and function of biomolecules that relate on the cause of variety diseases. To understand the mechanism of oxidative reactions, it is very important for analyze which molecules are involved in the oxidation process.
DMPO is the most popular spin-trapping reagent that traps radicals in protein and DNA samples. The DMPO-Protein or DMPO-DNA nitrone adducts are determined using a ELISA, Western Blotting, Mass spectorometry, Imaging, and so on. The most of commercialized DMPO contains impurities that cause high backgrounds. Thus, DMPO requires further purification steps before use it. The quality of Dojindo’s DMPO is well controlled and Dojindo’s DMPO doesn’t require any pre-purification process. There are no impurities to cause a background problem.
Principle of Immuno-spin Trapping Method
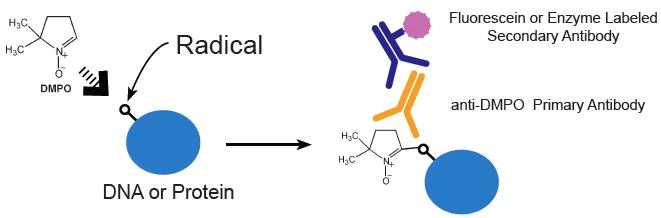
Anti-DMPO IgGs are available from Abcam
Example Protocol : Radical DNA Detection
► Referred Publication
Detection and imaging of the free radical DNA in cells–site-specific radical formation induced by Fenton chemistry and its repair in cellular DNA as seen by electron spin resonance, immuno-spin trapping and confocal microscopy.
Bhattacharjee S, Chatterjee S, Jiang J, Sinha BK, Mason RP., Nucleic Acids Res. 2012, 40, 5477-86
► Evaluation of radical DNA by ELISA
1. Extract DNA from RAW cells and dilute DNA to 5 μg/ml in PBS.
2. Add 25μl of DNA solution and 25μl of Reacti-Bind DNA coating solution in each well of the plate and incubatefor 2 Eh at 37°C.
3. Wash the wells once with washing buffer (PBS containing 0.05% non-fat dry milk and 0.1%Tween-20).
4. Block with blocking buffer (PBS containing 3% non-fat dry milk) for 2h at 37°C
5. Detect DMPO-DNA radical adduct with anti-DMPO and HRP-conjugated secondary antibody.
6. After three washes, add the Immobilon chemiluminescence substrate each well and measure the intensity of luminesscense.
► Another Aplication in this paper
• Cell Imaging
Example Protocol : Radical Protein Detection
► Referred Publication
Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling.
Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL., J Biol Chem. 2011, 286, 29098
► Evaluation of radical protein by cell imaging
1. Prepare of Bovine aortic endothelial cells (104 cells) in 35-mm dishes.
2. Add 10 μM Menadione and 50 mM DMPO and incuvate cells.
3. Wash the cells with PBS and fix them with 3.7% paraformaldehyde for 10 minutes.
4. Permeabilize the cells with 0.25% Triton X-100 in TBST(Tris buffered saline with Tween) for 10 minutes
5. Block the cells with 5% goat serum in TBST.
6. Visualize DMPO-protein radical adduct with anti-DMPO IgG and fluorescein labeled secondary antibody.
7. Analyze Protein radicals by fluorescent microscopy.
► Other Detections of Radical Protein
• Immunoblotting
• Mass Spectrometry
• Immunoprecipitation
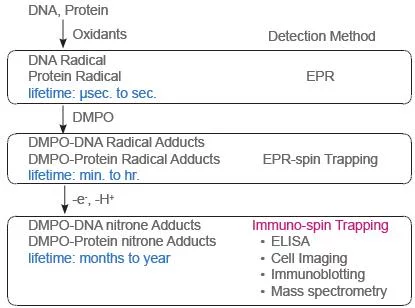
Radical Detection Scheme
References
1) S. Sankarapandi, et al., Evidence against the generation of free hydroxyl radicals from the interaction of copper,zinc-superoxide dismutase and hydrogen peroxide. J Biol Chem. 1999;274:34576-34583.
2) H. Li, et al., A pyrroline derivative of mexiletine offers marked protection against ischemia/reperfusion-induced myocardial contractile dysfunction. J Pharmacol Exp Ther. 2000;295:563-571.
3) H. P. Souza, et al., Quantitation of superoxide generation and substrate utilization by vascular NAD(P)H oxidase. Am J Physiol Heart Circ Physiol. 2002;282:H466-H474.
4) S. Kaewpila, et al., Manganese superoxide dismutase modulates hypoxia-inducible factor-1 alpha induction via superoxide. Cancer Res. 2008;68:2781-2788.
5) M. L. T. Teoh, et al., Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 2009;69:6355-6363.
6) Y. Song, et al., Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. PNAS. 2009;106:9725-9730.
Handling and storage condition
| Appearance: | Colorless liquid |
|---|---|
| Purity (GC): | ≥99.0% |
| -20oC, protect from light and moisture | |
| Density | 1.015g/ml at 25 oC |
|---|---|









