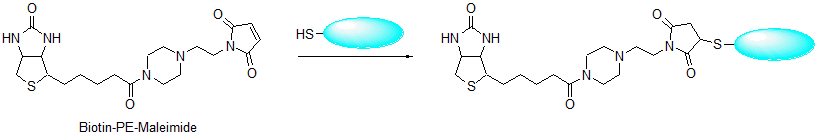
Biotin-PE-maleimide

Biotin Labeling Reagent
-
Product codeB592 Biotin-PE-maleimide
-
CAS No.374592-99-1
-
Chemical nameN-Biotinyl-N'-[2-(N-maleimido)ethyl]piperazine
-
MWC20H29N5O4S=435.54
| Unit size | Price | Item Code |
|---|---|---|
| 10 mg | B592-10 |
Description
The avidin-biotin system has many applications in immunology and histochemistry. The interaction between avidin and biotin is remarkably strong with a dissociation constant on the order of the 10-15 M. Biotin is usually added to primary or secondary antibodies such as anti-IgG and anti-IgM. After preparing the antigen-antibody complex with the biotin-labeled antibody, colorimetric or fluorometric detection of the antigen is performed using enzyme or fluorophore-labeled avidin or streptavidin. Maleimide biotins react with thiol compounds, such as proteins or peptides with sulfhydryl groups, at pH 7-7.5. Maleimide reacts with sulfhydryl group to create a thioether bond. Although other maleimide biotin reagents must be dissolved in DMSO, DMF, or alcohol, Biotin-PE-maleimide can be solubilized in PBS at pH 7.4 to prepare 2 mM solution without using an organic solvent. The reactivity of maleimide with sulfhydryl groups is higher than that of bromoacetamide, therefore the required concentration of maleimide biotin is much lower than that of bromoacetamide biotins. Stock solutions of Biotin-PEmaleimide and Biotin-PEAC5-maleimide in DMSO are stable for one year at -20ºC.
Technical info
1. Prepare 10 mM of the biotin labeling reagent using DMSO.
2. Prepare 100 μl of 1 mg per ml reduced IgG/ml buffer solution that does not contain any large molecules with SH groups. Reduced IgG can be prepared by TCEP (tricarboxyethylphosphine), DTT, or 2-mercaptoethylamine.
3. Add 1-5 μl of biotin labeling reagent DMSO solution to the IgG buffer solution and incubate at 37ºC for 1 hour.
4. Remove excess biotin labeling reagent using a gel column or a Filtration tube.
5. Prepare solutions for further experiments using an appropriate buffer, such as PBST (0.05% Tween 20/PBS).
References
1) S. Hashida, M. Imagawa, S. Inoue, K. H. Ruan and E. Ishikawa , "Mor Useful Maleimide Compounds for the Conjugation of Fab' to Horseradish Peroxidase throuth Thiol Groups in the Hinge", J. Appl. Biochem., 1984, 6, 56.
2) E. Ishikawa, M. Imagawa, S. Hashida, S. Toshitake, Y. Hamaguchi and T. Ueno, "Enzyme-labeling of Antibodies and their Fragments for Enzyme Immunoassay and Immunohistochemical Staining", J. Immunoassay, 1983, 4, 209.
3) H. -J. Friesen, P. Hermentin and P. Gronski, "Novel Maleimido-Biotins for the Selective Biotinylation of Sulfhydrils", Protides Biol. Fluids, 1987, 34, 43.
4) E. Ishikawa, S. Hashida, T. Kohno, T. Kotani and S. Ohtani, "Modification of Monoclonal Antibodies with Enzymes, Biotin, and Fluorochromes and Their Applications", Immunol. Ser., 1987, 33, 113.
5) R. B. del Rosalio and R. L. Wahl, "Disulfide Bond-targeted Radiolabeling : Tumor Specificity of a Streptavidine-biotinylated Monoclonal Antibody Complex", Cancer Res.(Suppl.), 1990, 50, 804S.
Handling and storage condition
| Appearance: | White to slightly yellow powder |
|---|---|
| Purity (HPLC): | ≧ 90.0 % |
| Solubility in Dimethyl sulfoxide: | To pass test (clear, colorless or slightly yellow) |
| -20°C |